A Guide to Chemical Mixing Scale Up! : From the Lab to the Factory
Understanding How Liquids Move
While intense chemical mixing is often seen as essential for chemical processes, a more nuanced view suggests its necessity is not universal; for very slow reactions where the chemistry itself is the bottleneck, aggressive agitation can be an unnecessary energy cost, and in some complex cases, it can even be detrimental by promoting unwanted side reactions. This concept is captured by the Damköhler number, which compares the mixing time to the reaction time; if chemical mixing is much faster than the reaction (Damköhler number less than 1), then stirring has little impact on the outcome.
However, for the majority of industrial applications where ingredients must be combined quickly and uniformly, especially at a large scale or with multiple phases (liquids, gases, solids), chemical mixing becomes a decisive factor. To grasp this, you must first understand how liquids move, which generally happens in one of two ways. Think of pouring honey slowly—it flows in smooth, parallel layers that slide past each other without mingling. This is called laminar flow, and while it’s predictable, it’s terrible for chemical mixing because the layers don’t want to combine.
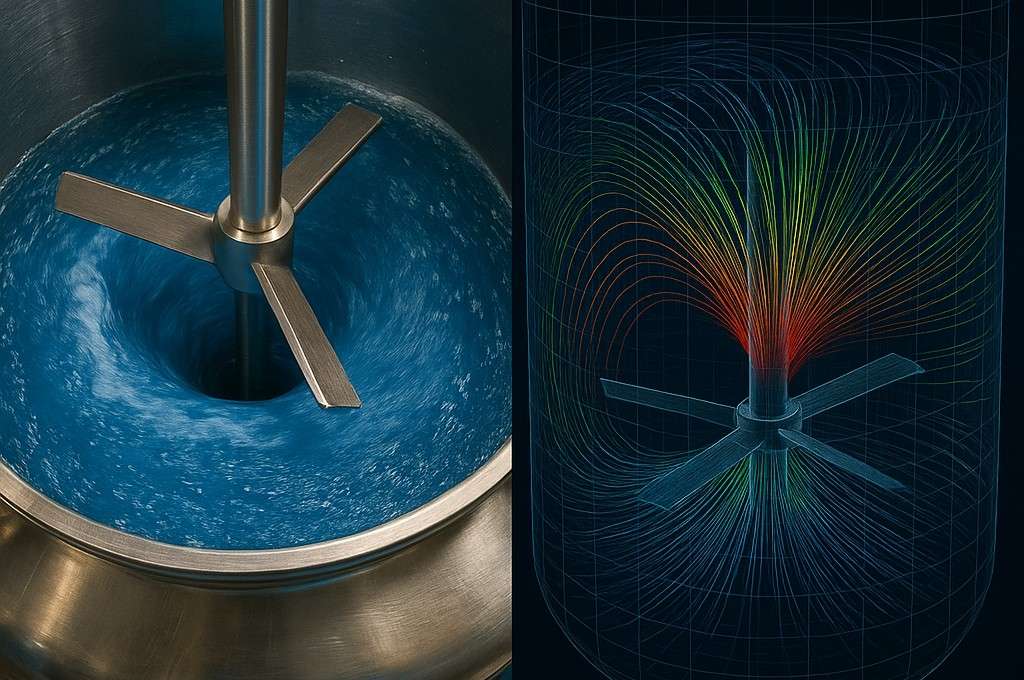
Now, imagine a chaotic, swirling river with rapids and whirlpools—that’s turbulent flow. It’s messy, but it’s fantastic for chemical mixing because it forces everything to crash together via convection, a process that is orders of magnitude faster than simple diffusion. In chemical plants, engineers almost always want turbulent flow to ensure ingredients are blended quickly and evenly, and that heat is distributed throughout the tank.

To improve dispersion and prevent the liquid from just spinning in a circle (vortexing), vertical plates called baffles are installed inside the tank. These plates break the circular flow and force the liquid to tumble from top to bottom, promoting much more efficient chemical mixing.
The sensitivity of chemical mixing is so high that even minor variations in experimental setup, such as the position of a vial on a stir plate, can lead to irreproducible results, a major challenge in process scale up. To figure out what kind of flow they have, they use a score called the Impeller Reynolds Number. A low score means calm, laminar flow, while a high score (usually above 10,000 for mixers) means you’ve hit the turbulent sweet spot where chemical mixing is most effective.
Measuring Mixer Performance
Not all mixers are created equal; they are specialized tools designed for different jobs. Engineers measure their performance using a few key numbers. The first is the Power Number (Np), which is like a drag rating for the mixer’s blade (called an impeller).
A high Power Number, often found on flat-bladed designs like the Rushton turbine, means the impeller creates a lot of drag and uses a lot of energy. This isn’t inefficient; it’s by design. That energy creates intense, localized cutting forces (shear), which are perfect for difficult tasks like dispersing gas bubbles into a liquid or blending thick, stubborn materials. On the other hand, a low Power Number, typical of sleek, propeller-like hydrofoil impellers, means the blade is highly efficient at moving large volumes of liquid around the tank with minimal energy.
This is ideal for gentle blending or just keeping solid particles suspended. This is a key consideration in any chemical mixing process, especially at a large scale. Another crucial metric is impeller tip speed, which is simply how fast the outer edge of the blade is moving.
This is important because some products, like delicate crystals or biological cells, can be damaged if the tip speed is too high. Conversely, for tasks like creating an emulsion, you need a very high tip speed to violently break droplets apart. Engineers use tables of recommended tip speeds for different jobs—from gentle blending (3-5 m/s) to high-shear homogenization (10-25 m/s)—to make sure they’re using the right tool for the job without destroying the product. Proper chemical mixing at any scale requires balancing these performance metrics.
The Challenge of Going Big
Taking a successful recipe from a small lab beaker to a giant factory tank is one of the most treacherous journeys in the chemical mixing industry, and simply making the equipment bigger is a proven recipe for failure. Physics doesn’t scale in a straight line, which creates predictable problems that must be managed with specific engineering and chemical solutions.
For instance, a common issue arises when using tall, skinny production tanks to save factory floor space. In these high-aspect-ratio tanks, a single impeller at the bottom, no matter how powerful, often can’t mix the liquid at the top, leaving a massive stagnant “dead zone”. This leads to batches where the product at the top is different from the product at the bottom. This exact scenario is a classic candidate for a Process Failure Mode and Effects Analysis (PFMEA), a formal risk assessment tool used to proactively identify and mitigate potential failures in the chemical mixing process. The PFMEA for this scale-up problem would look like this:
| Process Step | Potential Failure Mode | Potential Effect of Failure | Severity (S) | Potential Cause(s) | Occurrence (O) | Recommended Actions |
| Final Blending in Large Scale Tank | Vertical Stratification (uneven chemical mixing from top to bottom) | Entire batch fails quality specifications, leading to scrap or costly rework; customer complaints. | 10 | Use of a single impeller in a tall, narrow tank (High Aspect Ratio, H/T > 1.0). | 6 | Engineering Control: Redesign chemical mixing system for tall tanks to include a multi-impeller system to ensure top-to-bottom flow. |
To prevent this, the Control Plan, which documents how to manage the process, would mandate a specific engineering control:
| Process Step | Key Variable to Control | Specification / Tolerance | Measurement Method | Control Method | Reaction Plan |
| Final Blending | Blend Uniformity | Concentration difference between top and bottom samples < 2.0%. | Multi-point sampling (top, middle, bottom) followed by lab analysis (e.g., % solids). | Engineering Control: Use of validated multi-impeller system as per equipment design specification for tall tanks. | If uniformity fails, mix for an additional 15 minutes and re-sample. If it fails a second time, place batch on hold for technical review. |
Another common scale-up failure occurs when dispersing solids into liquids, like pigments in paint. In the lab, a high-speed mixer easily breaks apart pigment clumps. But in a large tank, after the intense chemical mixing stops, these tiny particles have a strong natural attraction and will clump back together, a process called flocculation. This causes major quality issues, such as a 20-40% loss in color strength, poor gloss, and pigment settling in the can over time. The solution is not just mechanical, but chemical. A PFMEA would identify the “Failure Mode” as “Particle Re-agglomeration,” with a key “Cause” being an “Incorrect order of addition.”
| Process Step | Potential Failure Mode | Potential Effect of Failure | Severity (S) | Potential Cause(s) | Occurrence (O) | Recommended Actions |
| Pigment Dispersion | Particle Re-agglomeration (Flocculation) after high-shear chemical mixing | Loss of color strength (20-40%); poor gloss; pigment settles in the can during storage, reducing shelf life. | 8 | Incorrect order of addition (dispersant added after shear); insufficient or incorrect type of dispersant used. | 5 | Procedural Control: Revise SOP and batch record to mandate dispersant is added before the high-shear step. |
The Control Plan would then specify a critical procedural control to ensure proper chemical mixing and stability:
| Process Step | Key Variable to Control | Specification / Tolerance | Measurement Method | Control Method | Reaction Plan |
| Pigment Dispersion | Order of Addition | Dispersant must be added to the liquid before the pigment powder is added and high-shear chemical mixing begins. | Operator verification and sign-off on batch record; Barcode scan confirmation in automated system. | Procedural Control: Batch record requires sequential sign-offs. Automated Control: Manufacturing Execution System (MES) prevents mixer from starting high-shear phase until dispersant scan is confirmed. | If order is incorrect, stop the process immediately. Place batch on hold. Notify supervisor and Quality department for disposition. |
High-Tech Tools and Special Mixers
To fight these complex problems in chemical mixing and scale-up, engineers have developed advanced tools. The most powerful is Computational Fluid Dynamics (CFD), which is essentially a virtual mixer inside a computer. CFD allows engineers to simulate the flow, temperature, and chemical concentrations inside a reactor before it’s even built. They can test dozens of different impeller designs, speeds, and tank shapes to find the best combination, spotting potential dead zones or hot spots on the screen and fixing them with a mouse click.
This saves enormous amounts of time and money compared to building and testing physical prototypes. For especially difficult materials, there is also specialized hardware. Beyond the coaxial mixer, which uses two concentric shafts for both bulk movement and high shear, other systems are used for large-scale chemical mixing.
For continuous processes, static mixers are often a better choice. These devices have no moving parts and are installed directly into a pipeline; they use a series of fixed, geometrically designed elements to force the fluid to split and recombine, achieving efficient chemical mixing with low energy and maintenance costs. For high-viscosity batch processes like those for adhesives or cosmetics, multi-shaft mixers or planetary mixers are employed. These combine different agitator types, such as a low-speed anchor and a high-speed disperser, to ensure thorough chemical mixing of thick materials that would otherwise not flow.
A Roadmap for Success
Successfully scaling up a chemical mixing process is a science, not guesswork. It requires a disciplined, step-by-step approach that combines careful planning with a deep understanding of the process. The journey to a larger scale often reveals surprising lessons, as seen in real-world failures. In one case, a process failed at a large scale because engineers focused on mixing solid particles when the real bottleneck was removing a gas—they had correctly solved the wrong problem.
In another counterintuitive case, a process making a pharmaceutical ingredient actually got worse with better chemical mixing. The more efficient chemical mixing sped up an unwanted side reaction that created impurities, proving that “more chemical mixing” isn’t always the answer; the “right chemical mixing” is. The key takeaway from decades of experience is to always assume chemical mixing could be a problem until you prove it isn’t. True success in any chemical mxing scale-up depends on clear communication between chemists and engineers, treating scale-up as a critical part of development from the very first day in the lab, and prioritizing a scientific understanding of the process over simplistic rules of thumb.
Source: https://thebulklab.com